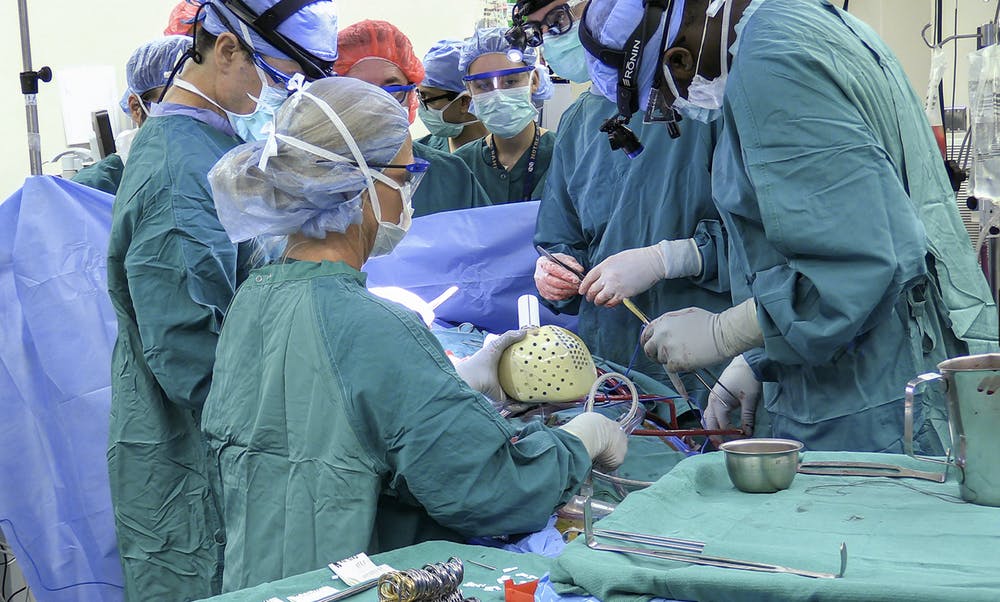
Carmat artificial heart being implanted at Duke University in July, 2021
Although left ventricular assist devices have been around for decades, French biotech startup Carmat has made waves with its new artificial heart. This total heart prosthesis may become the second best option to receiving a donor heart (and the supply of hearts is extremely limited.) The Carmat heart is designed to last for several years but is currently being marketed in Europe and tested in the United States only as a six month bridge to transplant.
As of October, 2021 three Carmat hearts had been implanted in patients in the United States including the first at Duke University and two (the most recent - a woman) at the University of Louisville/ Jewish Hospital. These implants are part of an FDA investigational device exemption (IDE) trial in America.
The first patient, a 39 year old man named Matthew Moore, received a Carmat heart at Duke in July, 2021. Here the cardiothoracic team at Duke describes the operation and the patient. Now, three months later he has been discharged from the hospital.
The heart has been approved for patients in Europe with end stage biventricular failure under a CE Mark since early 2021.
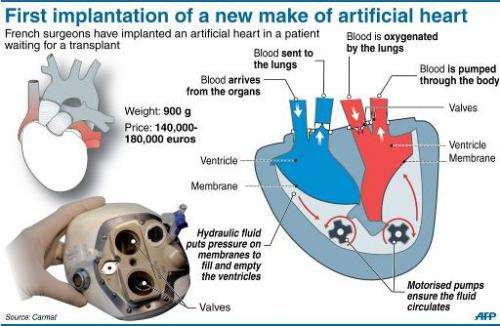
compartments and blood flow
Any artificial heart should possess the following features.
The Carmat heart has several breakthrough features including 1) biologic membrane use in all surfaces that contact blood, 2) pulsatile blood flow provided by two electrohydraulic pumps, and 3) electronic autoregulation of pulse rate and ventricular pressures. Autoregulation performance is described in this October, 2021 publication. The heart is self-contained and requires only an electric cable to an external controller and battery.
Blood flowing through the heart only contacts four bovine heart valves and bovine pericardial membrane (lining both ventricles.) These natural materials have been long established in cardiothoracic surgery as a means of reducing blood clots and trauma to cells (as opposed to artificial materials like dacron). Furthermore, autoregulation and pulsatile flow are important aspects in aligning cardiac output with exercise and in preventing pulmonary hypertension and overload. Here is a beautiful and detailed animation of the Carmat heart (branded under the trademark Aeson.)
I've followed this development for the past decade as Carmat has implanted approximately twenty patients, mainly in Europe, in the course of testing and improving the device. In 2018 the company announced two automated assembly plants close to Paris capable of producing eight hundred hearts per year. In October, 2021 Investment Banking Firm Ladenburg Thalmann hosted a comprehensive video seminar on Carmat's accomplishments. This seminar was superb. You can download my transcription of this 90 minute conference video here. (See your download folder.)
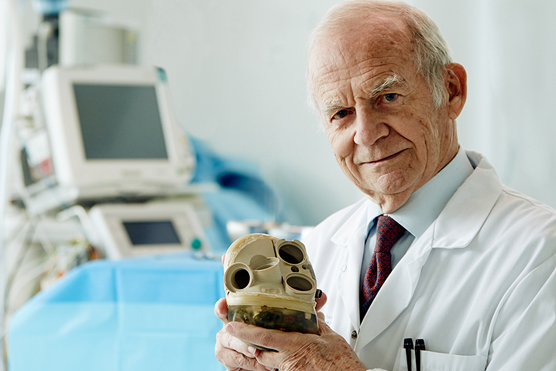
Founder of Carmat, Professor Alain Carpentier, with the Carmat total artificial heart
The Carmat heart is the creation of brilliant cardiac surgeon/ bioengineer Alain Carpentier and has benefited from generous intellectual and financial support from the European aerospace giant Airbus as well as from government and VC financing.
A variety of artificial hearts and left ventricular assist devices (LVADs) have been developed over the past fifty years.
For a bridge to transplant the Syncardia artificial heart has been the best solution for many patients awaiting a donor heart. It's been implanted in more than 1400 patients enabling many to go home. Following bankruptcy proceedings in 2016, Syncardia was acquired by Versa Capital Management.
Another maker of LVADs was Heartware, acquired by Medtronic in 2016 for over a billion dollars. See the inspiring 2016 story of this young athlete kept alive with his Heartware LVAD. Unfortunately, in 2021 Medtronic had to discontinue the device due to an increased incidence of strokes and death.
Another LVAD maker I've followed was Thoratec, which was founded in 1976, and acquired by St. Jude Medical in 2015. The St. Jude effort was, in turn, acquired by Abbott in 2017. See Abbott's Heartmate 3 LVAD here.
Here is former Vice President Dick Cheney "arrestingly" chatting after his battery-powered LVAD was implanted but before his 2012 heart transplant.
In this remarkable 2018 video we get a 360 degree view of a heart transplant including midline sternotomy and insertion of the donor heart.
I frequently hear stunning bioengineering breakthroughs at Stanford from visiting investigators and our own faculty in biomechanical engineering, material science, and regenerative medicine (of course, during the pandemic via Zoom.)
This update was posted on 13 Oct 2021
(Substantive comments may be emailed to bob AT bobblum DOT com. With your permission excerpts may be posted here.)
October, 2021: Thanks to the following who've conveyed their praise of and brief comments on this article: Patrick G, James P, Jay S, Arafat M, and Andrew L.
21 October 2021: Electro-optics engineer Dennis Hancock writes:
How about including the following diagram from Carmat's website:
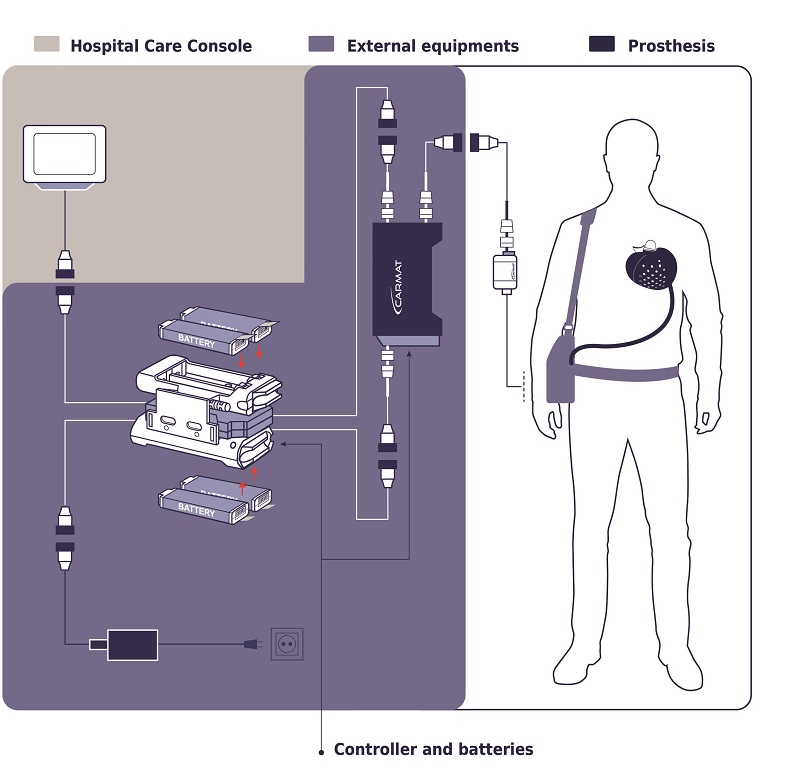
Components in the purple box above include the battery and controller to the right that is carried in a pouch when the patient is awake. Also shown is the controller and batteries that are non-portable for nighttime charging and control.
The portion of the external controller and battery system that the patient carries weighs 4 kilograms and lasts for 4 hours of operation.
I (RLB) respond:
Thanks so much, Dennis. The diagram is somewhat confusing. It's easiest to understand if you focus on the connection plugs that help identify the four different subsystems in the purple box in the diagram (the external stuff either carried by the patient during the day or plugged in at night on his nightstand.
Dennis Hancock continues ...
Here are a few more items of an engineering performance nature that you might include.
The auto-regulation feature controls the heart pumping rate from 78 to 128 beats/min. The volume of blood pumped in the left ventricle is 4.3 to 7.3 liters/min. The volume of blood pumped in the right ventricle is 4.2 to 7.2 liters/min. (Source: the 2021 article published by the Carmat team on autoregulation. )
To put a heart’s performance in perspective, pumping 5 liters/ min (average) over a year results in the transferrance of 525,000 liters/year, or 138,849 gallons/year which is around 21 percent the volume of an Olympic sized swimming pool.
This page in The Physics Handbook shows the (utilized) Power of a Human Heart. Estimates of the power output of the heart range from 1.3 watts to 5 watts. Note that the chemical energy from ATP required as input is much greater — about 13 watts.
Also note the description of the design of the Carmat Aeson heart in Wikipedia.
In Carmat's design, two chambers are each divided by a membrane that holds hydraulic fluid on one side. A motorized pump moves hydraulic fluid in and out of the chambers, and that fluid causes the membrane to move; blood flows through the other side of each membrane. The blood-facing side of the membrane is made of tissue obtained from a sac that surrounds a cow's heart, to make the device more biocompatible. The Carmat device also uses valves made from cow heart tissue and has sensors to detect increased pressure within the device. That information is sent to an internal control system that can adjust the flow rate in response to increased demand, such as when a patient is exercising. This distinguishes it from previous designs that maintain a constant flow rate.
RLB responds: Again, thanks for your contributions, Dennis
10 Jan 2022: Pig to Human Heart Transplant: Success (so far): Day 3. Undertaken at Univ. of Maryland (Baltimore) on 7 Jan 2022.
A stunning development. I'm guessing the logistics didn't permit use of the Carmat heart, which would've conferred greater probability of one year survival.
(Substantive comments may be emailed to bob AT bobblum DOT com. With your permission excerpts may be posted here.)